GMP-Compliant Products & Services
Explore our range of high quality, GMP-compliant Products & Services to streamline your clinical translation.
- Reliable, high quality and GMP-compliant ancillary materials for your Advanced Therapy Medicinal Products (ATMP) research.
- Designed from Bench to Bedside: Streamlined preclinical to clinical translation.
- FDA-registered with Drug Master Files (DMF) available
- Fully documented and traceable with all required documentation available on request.
- Stable for storage and shipping with International Council for Harmonisation (ICH) stability studies supporting enzyme storage and shipping conditions.
- Supported by an ISO9001 Certified company as AMSBIO is certified in the UK, The Netherlands and is working towards certification the United States.
Disclaimer: AMSBIO's GMP-compliant Products & Services are not pharmaceuticals/therapeutic products, and are only intended for manufacture of investigational ATMPs. They are not suitable for direct administration to humans.
Cell Culture Media
Cell culture media is a vital ancillary material for the development of cell therapies, and our StemFit® medium offers superior maintenance and colony expansion of pluripotent stem cells (iPSCs and ESCs).
StemFit® Range
Developed in collaboration with Nobel-prize winning scientist Prof. Shinya Yamanaka, StemFit® is an animal-origin free, chemically-defined and clinically relevant medium. Two variants are available in a GMP-compliant formulation, ideal for pre-clinical and clinical research.
- StemFit Basic04 CT GMP-Grade: One bottle formulation with bFGF included for reduced handling and increased cost efficiencies
- StemFit Basic03 GMP-Grade: Two bottle formulation with no bFGF for greater customization within the facility to meet your needs
Benefits:
- Guaranteed Quality & Safety: StemFit Basic03 CT GMP-grade is manufactured under GMP guidelines in the USA. StemFit Basic04 CT GMP-grade is manufactured in Japan, under GMP guidelines that follow the Ajinomoto System of Quality Assurance (ASQUA), based on ISO 9001
- Avoid regulatory delays by using animal-origin free and clinically relevant cell culture media early in your ATMP research.
- Confirmed by the PMDA in Japan to be eligible for use in cell therapy.
- Superior performance compared to other commercially available medias.
*Japan equivalent to FDA.
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| StemFit ® Basic03 GMP grade (animal-origin free, defined medium for human pluripotent stem cell culture, GMP grade) | Liquid A: 400ml / Liquid B: 100ml | View | |
| StemFit® Basic04 Complete GMP Grade (animal-origin free, defined medium for human pluripotent stem cell culture, including 80ng/ml bFGF, GMP Grade) | 500 ml | View |
Extracellular Matrices
Extracellular Matricies (ECMs) are used for high throughput production of iPSCs to support growth and differentiation. ECMs can be of variable quality and so GMP-grade ECMs ensure reproducibililty and consistency for quality control.
iMatrix
iMatrix-511MG is for the maintenance and expansion of iPSCs. A highly purified and refined product of human recombinant laminin-511 E8 fragment expressed by CHO-S cells. iMatrix-511 easily enables extended culture of human pluripotent stem cells and has swiftly established itself as the gold standard for stem cell culture.
iMatrix-511MG meets the Standards of Biological Ingredients set by the Pharmaceutical and Medical Devices Agency (PMDA) – the Japanese government agency which is equivalent to the FDA in the USA. Their regulation is one of the strictest in the world and iMatrix-511MG not only fulfils regulatory requirements, it has since been used for a number of high-profile clinical applications in Japan: iMatrix-511MG is a GMP compatible and GMP adaptable resource
To enquire about iMatrix™-511 MG, please email us at [email protected].
CollaFibR™ Scaffolds
3D cell cultures offer a more accurate representation of in-vivo conditions when studying cell and tissue models. Using dry-spinning technology, the CollaFibR™ scaffold consists of highly uniform and regulatory friendly collagen fibers to closely mimic the biomechanical and biochemical characteristics of natural collagen scaffolds.
In addition, should the premade scaffolds not be suitable, the automated manufacturing system has a high level of control and versatility, allowing us to create customized CollaFibR™ scaffolds tailored to meet your specific manufacturing needs. We can control the thicknesses, porosities, and alignment of our scaffolds with a high batch-to-batch consistency. Please feel free to contact us at [email protected] to discuss your specific needs.
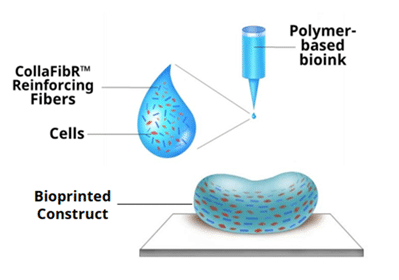
μCollaFibR™- Additive for Bioinks and Hydrogels
μCollaFibR™ are engineered dry-spun 50 μm collagen fibers that increase the shape fidelity and biological relevance of bioprinted constructs. With exceptional chemical stability and ultra-fine 1-2 μm diameters, μCollaFibR™ is universally compatible with bioprinting materials and modalities.
- Produced using GMP bovine type I collagen, and resemble natural collagen fiber structures
- Increases mechanical strength and modulus of hydrogels in extension and compression
- Improves shape retention/durability for at least 28 days in cellular constructs
- Improves cellular viability and functionality within bioprinted cell constructs
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| CollaFibR Scaffold: No FITC/ with coverslip (Cross-Hatched Fibers) | Each (Individually Packaged Ring Insert, to fit 12 well plate) | View | |
| CollaFibR Additive (for Bioinks and Hydrogels). No FITC/ short fibers. | 25 mg | View | |
| CollaFibR Additive for Bioinks and Hydrogels. No FITC/ short fibers. | 10 mg | View | |
| CollaFibR Scaffold: No FITC/ no coverslip (Cross-Hatched Fibers) | 12x (Individually Packaged Ring Inserts, to fit 12 well plate) | View | |
| CollaFibR Scaffold: No FITC/ no coverslip (Cross-Hatched Fibers) | Each (Individually Packaged Ring Insert, to fit 12 well plate) | View | |
| CollaFibR Scaffold: No FITC/ with coverslip (Cross-Hatched Fibers) | 12x (Individually Packaged Ring Inserts, to fit 12 well plate) | View | |
| Fluorescent CollaFibR Additive for Bioinks and Hydrogels. FITC Tag/ short fibers | 25 mg | View | |
| Fluorescent CollaFibR Scaffold: FITC tag/ with coverslip (Cross-hatched Fibers) | 12x (Individually Packaged Ring Inserts, to fit 12 well plate) | View | |
| Fluorescent CollaFibR Scaffold: FITC tag/ with coverslip (Cross-hatched Fibers) | Each (Individually Packaged Ring Insert, to fit 12 well plate) | View | |
| Fluorescent micro-CollaFibR (TM) Additive for Bioinks and Hydrogels. FITC Tag, short fibers | 10 mg | View |
Recombinant Proteins
Directed differentiation of pluripotent stem cells into a variety of specialized tissue types is stimulated by the addition of recombinant proteins to cell culture media. Our GMP-grade StemFit Purotein® options are ideal for the development of differentiated cell types for ATMPs. StemFit Puroteins® are also highly compatible with our StemFit® medium!
StemFit Purotein® Recombinant Proteins
StemFit Purotein® Activin A and bFGF recombinant proteins are manufactured to GMP guidelines and have been approved by the PMDA* in Japan for ATMP manufacture.
bFGF is a growth factor used in the expansion of iPS/ES cells, and both bFGF and Activin A are used to direct the differentiation of iPS/ES cells towards cells of the liver, pancreas, or kidney.
Benefits:
- Animal-origin free.
- High purity and performance.
- Highly compatible with StemFit® cell culture media
- Reliable lot-to-lot consistency.
- Ready-to-use frozen formulation, simplifying your protocols & reducing the risk of bacterial contamination.
Cryopreservation Media
High-viability and streamlined cryopreservation of stem cells for storage and shipping is crucial for the successful commercialization of ATMPs. AMSBIO offers a wide range of GMP-grade cryopreservation solutions suitable for pre-clinical and clinical applications.
STEM-CELLBANKER®
A chemically-defined, animal-free stem cell cryopreservation solution manufactured in compliance with JPN, EU, US, and PIC/S GMP guidelines.
Benefits:
- Clinically relevant - contains only European or US Pharmacopoeia graded ingredients.
- Optimized for storage of ES/iPSCs and other fragile cell types, it is an optimal choice for storage of cells developed for cell therapy applications.
- Drug Master File registered with the FDA, for a streamlined clinical translation.
- Validated by high profile laboratories for cryopreservation and recovery of whole organoids, with high viability rates. Find out more here.
STEM-CELLBANKER® DMSO-free
GMP-grade STEM-CELLBANKER® is also available in a DMSO-free formulation, for clinical applications where DMSO is an unsuitable freezing agent.
STEM-CELLBANKER® EX
Expanding the possibilities of cell therapy, STEM-CELLBANKER® EX is a GMP-compliant, chemically defined, sterile, & xeno-free cryopreservation media you can rely on for safe & easy storage of your ATMP.
- Composed of ingredients that are already approved in the manufacture of cell therapy excipients for intravenous injection.
- Optimized for applications in regenerative medicine: no requirement for washing of the cryopreservation solution upon delivery of the cell therapy product.
- Drug Master File registered with the FDA - CBER.
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| STEM-CELLBANKER - GMP Grade (100ml) (formerly 11890) | 100 ml | View | |
| STEM-CELLBANKER - GMP Grade (20ml) (formerly 11897) | 20 ml | View | |
| STEM-CELLBANKER - GMP Grade (4 x 20 ml) | 4 x 20 ml | View | |
| STEM-CELLBANKER DMSO FREE - GMP Grade (100ml) (formerly 11890F) | 100 ml | View | |
| STEM-CELLBANKER DMSO FREE - GMP Grade (20ml) (formerly 11897F) | 20 ml | View | |
| STEM-CELLBANKER DMSO FREE - GMP Grade (4 x 20 ml) | 4 x 20 ml | View | |
| STEM-CELLBANKER EX - GMP Grade (100ml) (formerly 11890EX) | 100 ml | View |
Custom CELLBANKER®
Custom CELLBANKER® is the ideal GMP-grade cryopreservation solution for scaling up from research to manufacturing. Tailor the formulation, volume, and container type to your needs to ensure seamless integration into large-scale cell therapy product manufacturing and storage to match your workflow and regulatory requirements. Contact us on [email protected] to discuss your cryopreservation requirements, or fill in our Custom Request form.
Dissociation Enzymes
Efficient and reliable cell detachment and tissue dissociation using enzymes is crucial for cell viability and successful downstream applications of the dissociated cells. Therefore, dissociation enzymes are key ancillary materials in ATMP development.
In partnership with Nordmark Pharma GmbH, AMSBIO provides superior GMP-compliant Collagenase and Neutral Protease with high quality and reliable lot-to-lot consistency, for your ATMP research.
Benefits:
- Optimal for applications with GMP requirements e.g. tissue engineering and cell therapy.
- Superior quality enzymes with reliable lot-to-lot consistency for your ATMP research.
- Cost effective - Research-Grade formats with equivalent enzymatic activities are available for protocol optimisation.
- ICH stability studies* support storage and shipping at room temperature (+2 to +8 °C) without compromised activity or quality.
- Animal-origin free enzymes are available, for the highest possible safety standard.
- All required documentation is available upon request.
*ongoing ICH study for N0003855.
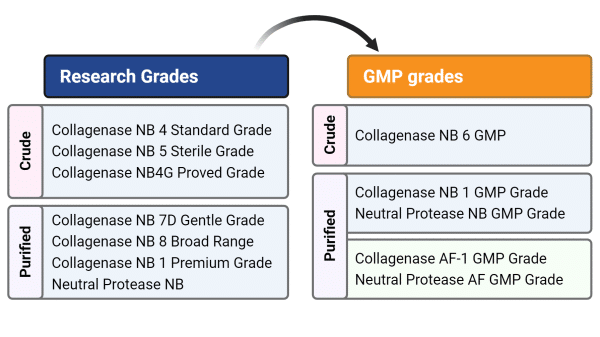
Collagenase and Neutral Protease NB
GMP-compliant Collagenase and Neutral Protease NB are an optimal solution for isolation and passaging of a broad range of cells dedicated for tissue engineering and transplantation into humans.
High Safety Standards:
- TSE (transmissible spongiform encephalopathy) safety of the fermentation product is certified by the EDMQ.**
- Endotoxin tested.
- ICH stability & virus validation studies.
** fermentation medium for Collagenase and Neutral protease NB production contains raw materials of bovine origin. EDMQ = European Directorate for the Quality of Medicines & Healthcare.
| Name | Datasheet | Packsize | Order |
|---|---|---|---|
| Collagenase AF-1 GMP Grade | >= 150 U (PZ) | View | |
| Collagenase AF-1 GMP Grade | >= 2000 U (PZ) | View | |
| Collagenase NB 1 GMP Grade | >= 2000 U (PZ) | View | |
| Collagenase NB 6 GMP Grade | 1 g | View | |
| Collagenase NB 6 GMP Grade | 100 mg | View | |
| Neutral Protease AF GMP Grade | >= 100 U (DMC) | View | |
| Neutral Protease NB GMP Grade | >= 100 U (DMC) | View |
Animal-free Collagenase and Neutral Protease
Nordmark AF enzyme manufacturing process is completely free of any animal-based components, ruling out TSE concerns and reducing the risk of regulatory hurdles.
Instead, Collagenase AF-1 GMP Grade and Neutral Protease AF GMP Grade are derived from a fermentation process using a plant-based medium, providing the highest level of safety!
Our AF enzymes are highly active and designed to effectively isolate very sensitive cells from tissues such as liver, pancreas, and solid tumours.

Citations
STEM-CELLBANKER
Cell response analysis in SARS-CoV-2 infected bronchial organoids.
Sano, E., Suzuki, T., Hashimoto, R., Itoh, Y., Sakamoto, A., Sakai, Y., . . . Takayama, K. (2022). Communications Biology, 5(1), 516.
A protocol to differentiate nociceptors, mechanoreceptors, and proprioceptors from human pluripotent stem cells.
Saito-Diaz, K., & Zeltner, N. (2022). STAR Protocols, 3(2), 101187.
Establishment of Organoids From Human Epithelioid Sarcoma With the Air-Liquid Interface Organoid Cultures.
Wakamatsu, T., Ogawa, H., Yoshida, K., Matsuoka, Y., Shizuma, K., Imura, Y., . . . Takenaka, S. (2022). Front Oncol, 12, 893592.
Phenotyping clonal populations of glioma stem cell reveals a high degree of plasticity in response to changes of microenvironment.
Innes, J. A., Lowe, A. S., Fonseca, R., Aley, N., El-Hassan, T., Constantinou, M., . . . Brandner, S. (2022). Laboratory Investigation, 102(2), 172-184.
Inflation-collapse dynamics drive patterning and morphogenesis in intestinal organoids.
Tallapragada, N. P., Cambra, H. M., Wald, T., Keough Jalbert, S., Abraham, D. M., Klein, O. D., & Klein, A. M. (2021). Cell Stem Cell, 28(9), 1516-1532.e1514.
On-chip 3D neuromuscular model for drug screening and precision medicine in neuromuscular disease.
Osaki, T., Uzel, S. G. M., & Kamm, R. D. (2020). Nature Protocols, 15(2), 421-449.
Xeno-Free Reprogramming of Peripheral Blood Mononuclear Erythroblasts on Laminin-521.
Skorik, C., Mullin, N. K., Shi, M., Zhang, Y., Hunter, P., Tang, Y., . . . Schlaeger, T. M. (2020). Current Protocols in Stem Cell Biology, 52(1), e103.
Cardiac hypertrophy in a dish: a human stem cell based model.
Johansson, M., Ulfenborg, B., Andersson, C. X., Heydarkhan-Hagvall, S., Jeppsson, A., Sartipy, P., & Synnergren, J. (2020). Biology Open, 9(9), bio052381.
STEM-CELLBANKER DMSO-free
Longitudinal single-cell RNA-seq analysis reveals stress-promoted chemoresistance in metastatic ovarian cancer.
Zhang, K., Erkan, E. P., Jamalzadeh, S., Dai, J., Andersson, N., Kaipio, K., . . . Vähärautio, A. (2022). Science Advances, 8(8), eabm1831.
Comparison of islet isolation result and clinical applicability according to GMP‐grade collagenase enzyme blend in adult porcine islet isolation and culture.
Kwak, K., Park, J. k., Shim, J., Ko, N., Kim, H. J., Lee, Y., . . . Choi, K. (2021). Xenotransplantation (Københaven), 28(4), e12703-n/a.
Comparison of Islet Characterization from Use of Standard Crude Collagenase to GMP-Grade Collagenase Enzyme Blends in Preweaned Porcine Islet Isolations.
Corrales, N., Park, S., Lau, H., Xu, I., Luong, C., Rodriguez, S., . . . Lakey, J. R. T. (2020). Cell Transplantation, 29, 0963689720977835