Cultured Meat
A New Era in Food Technology
Advances in cell culture, scaffolding and bioprinting have enabled the production of genuine meat, called cultured meat, from animal cells without the need for animal slaughter or the environmental costs of raising livestock.
AMSBIO products are supporting the ground-breaking research into this exciting new area of food technology, and aiding the development of new cultured meat products.
What is Cultured Meat?
- Cultured meat is made by growing animal cells in an artificial environment, with the aim of producing meat resembling the texture, flavour and nutritional value of conventional meat, without the ethical and environmental costs of traditional farming.
- It is predicated that the use of this new technology could reduce the environmental impact of meat consumption by around 90% (Sinke et al., 2023).
- The sustainability benefits of cultured meat include the potential reduction in air pollution, deforestation, water use, water contamination, biodiversity loss, antibiotic resistance and foodborne illnesses.
- The overall goal is to mass produce cultured meat products to supply the increasing global demand for meat, whilst reducing the impact of meat production on the environment.
Comparison with Conventional Meat Production

How is Cultured Meat made?
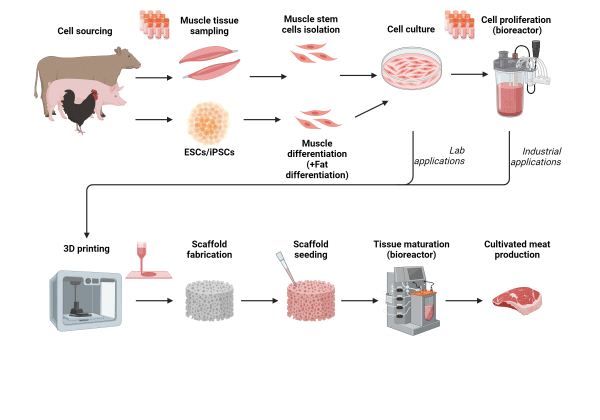
Cultured meat manufacturing starts with identifying a suitable source of cells that can differentiate into muscle and fat cells. Most commonly this is through using primary cells (multipotent) isolated from tissues of livestock animals but alternatively pluripotent stem cells can be differentiated into muscle/fat specific cell types. A cell line (e.g. myogenic cells) is established and the cells are proliferated in a bioreactor to generate a large source of starting material. The cells can then be differentiated to form mature muscle cell types and subsequent processing can produce unstructured meat products e.g. beef mince. Alternatively, the cells can be co-cultured with other cell types (e.g. adipose stem cells) in an edible scaffold to enable differentiation and formation of structured meat products e.g. steak. Formation of structured meat products can also be achieved by bio-printing of muscle and fat cells to resemble more complex tissue structures.
Current Research and Development
- AMSBIO offers vital products for pioneering cultured meat research, including iMatrix-511 Laminin ECM, cryopreservation media and skeletal muscle differentiation kits.
- We also offer a vast biorepository containing animal DNA, RNA and tissue to act as positive control to compare samples, along with ELISA kits for quantification of specific proteins, such as collagen.
Different Categories of AMSBIO products previously used in the Cultured Meat Industry:
- Cell culture: iMatrix-511 Recombinant Laminin E8 Fragments, MAPTrix™ Recombinant Animal Free ECM, Skeletal muscle differentiation kit, Recombinant proteins and Lentiviral particles
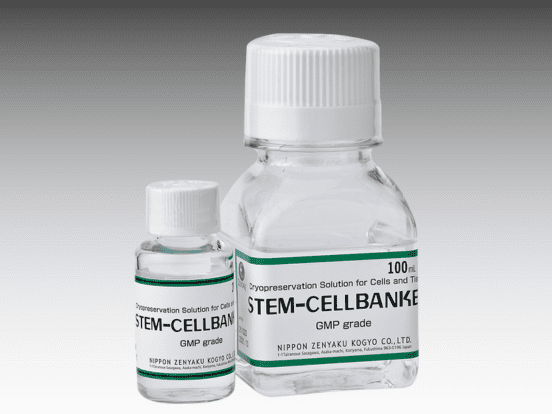
- Cryopreservation media to store cells for reference or future use: CELLBANKER, STEM-CELLBANKER, CELLOTION
- Standards and kits to test cell identity and maturity compared to in vivo: Biorepository, Assay kits
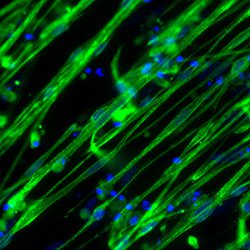
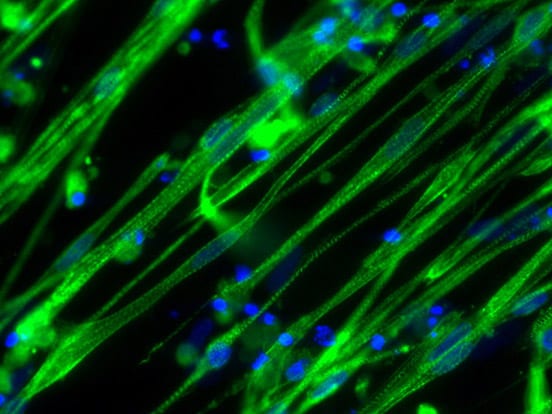
Case Study: Growth of Pig Stem Cells on iMatrix-511 silk as recombinant ECM
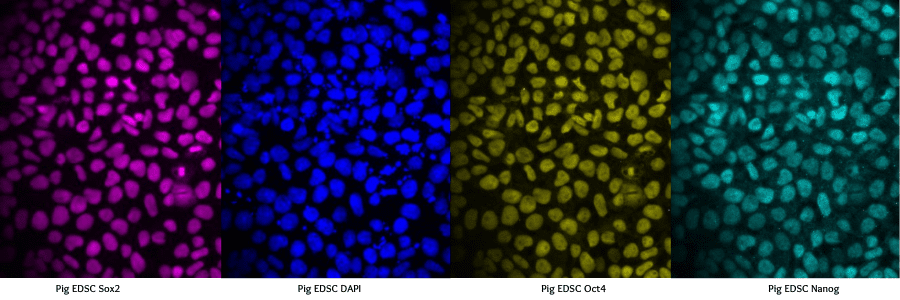
The Commercial Landscape of Cultured Meat
- 2023 was a milestone year for the advancement of cultivated meat with the approval of two companies, GOOD Meat and Upside foods, to sell lab-grown meat to restaurants in the United States. In July 2023, the Netherlands became the first European country to allow cultivated meat and seafood tasting. As of January 2024, Israel became the third country to advance the approval of cultivated meat sales.
- Cultivated meat and seafood companies have raised $3.1 billion since 2013 with significant growth in investments (83%) in the last 3 years.
- Many new cultured meat ventures have emerged all over the world in recent years! Start-up companies are not only developing cultured meat products, but also the materials and equipment necessary for commercialisation.
- Despite this growth, difficulties with scaling up for global manufacturing, and spread of misinformation remain obstacles for the commercialisation of cultivated meat. Consumer education, understanding, and adoption are still in the early stages. And the work to secure increased government support for the industry continues.

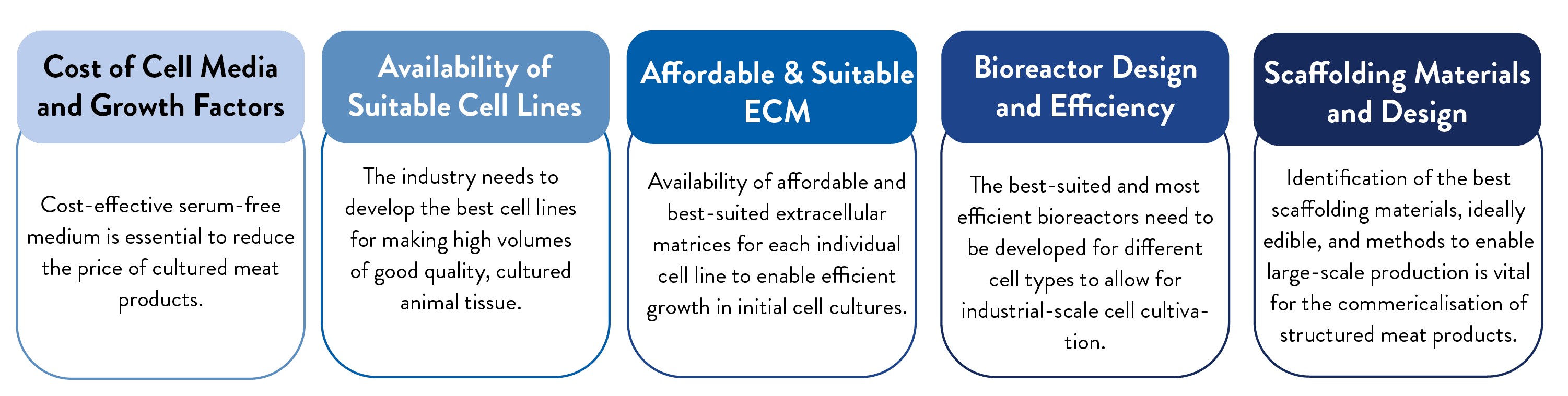
Challenges to Commercialisation
At scale, cultivated meat could enable a shift toward less resource-intensive ways of producing protein and increased food security. But first, the industry must overcome challenges, such as disinformation and proposed bans or restrictions on cultivated meat, in the United States and around the world. Additionally, to scale production for mass consumption, there are complex challenges in 5 main areas that need to be solved:
The Future
• The next step is to reduce costs of key components to scale up production of cultured meat to an industrial scale for mass consumption.
• Obtaining regulatory approval from governments and regulatory bodies is essential for the success of cultured meat.
Featured Citations
Pluripotent stem cells related to embryonic disc exhibit common self-renewal requirements in diverse livestock species.
Kinoshita, M., Kobayashi, T., Planells, B., Klisch, D., Spindlow, D., Masaki, H., ... Alberio, R. & Smith, A., (2021), Development, 148(23), dev199901.
Citing iMatrix Recombinant Laminin 511-E8 fragments
Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat.
Stout, A. J., Mirliani, A. B., White, E. C., Yuen, J. S. K., & Kaplan, D. L. (2021), bioRxiv, 2021.2005.2028.446057
Citing iMatrix Recombinant Laminin 511-E8 fragments
Engineering carotenoid production in mammalian cells for nutritionally enhanced cell-cultured foods.
Stout, A. J., Mirliani, A. B., Soule-Albridge, E. L., Cohen, J. M., & Kaplan, D. L. (2020), Metabolic Engineering, 62, 126-137.
Citing iMatrix Recombinant Laminin 511-E8 fragments
Extracellular heme proteins influence bovine myosatellite cell proliferation and the color of cell-based meat.
Simsa, R., Yuen, J., Stout, A., Rubio, N., Fogelstrand, P., & Kaplan, D. L. (2019), Foods, 8(10).
Citing iMatrix Recombinant Laminin 511-E8 fragments
The amount range of membrane cholesterol required for robust cell adhesion and proliferation in serum-free condition.
Takii, S., Wu, J., & Okamura, D. (2021), bioRxiv, 2021.2010.2021.465296
Citing iMatrix Recombinant Laminin 511-E8 fragments
Harvest of quality-controlled bovine myogenic cells and biomimetic bovine muscle tissue engineering for sustainable meat production.
Takahashi, H., Yoshida, A., Gao, B., Yamanaka, K., & Shimizu, T. (2022), Biomaterials, 287, 121649.
Citing iMatrix Recombinant Laminin 511-E8 fragments.
Isolation and Characterization of Tissue Resident CD29-Positive Progenitor Cells in Livestock to Generate a Three-Dimensional Meat Bud.
Naraoka, Y., Mabuchi, Y., Yoneyama, Y., Suto, E. G., Hisamatsu, D., Ikeda, M., . . . Akazawa, C. (2021), Cells, 10(9), 2499.
Citing CELLBANKER 1 (from NIPPON ZENYAKU KOGYO)
Kakehi, R., Yoshida, A., Takahashi, H. and Shimizu, T. (2023). Frontiers in sustainable food systems, 7. doi:https://doi.org/10.3389/fsufs.2023.1023057.
Citing CELLBANKER 1 and iMatrix Recombinant Laminin 511-E8 fragments
Sources and Further Reading
2023 State of the Industry Report: Cultivated meat and seafood
Good Food Institute, (2023)
The Science of Cultivated Meat Good Food Institute, (2021) and Cultivated meat Good Food Institute (2024)
Environmental Impacts of Cultured Meat Production.
Tuomisto, H. L., & Teixeira de Mattos, M. J. (2011), Environmental science and technology, , 45(14), 6117-6123.
Scientific, sustainability and regulatory challenges of cultured meat.
Post, M. J., Levenberg, S., Kaplan, D. L., Genovese, N., Fu, J., Bryant, C. J., . . . Moutsatsou, P. (2020), Nature Food, 1(7), 403-415.
Tissue Engineering for Clean Meat Production.
Ben-Arye, T., & Levenberg, S. (2019), Frontiers in sustainable food systems, 3.
Trends and ideas in technology, regulation and public acceptance of cultured meat.
Guan, X., Lei, Q., Yan, Q., Li, X., Zhou, J., Du, G., & Chen, J. (2021), Future Foods, 3, 100032.
Ex-ante life cycle assessment of commercial-scale cultivated meat production in 2030
Sinke, P., Swartz, E., Sanctorum, H. et al. (2023). Int J Life Cycle Assess 28, 234–254. https://doi.org/10.1007/s11367-022-02128-8
Advances and Challenges in Cell Biology for Cultured Meat
Martins, B., Bister, A., Richard G.J. Dohmen, Maria Ana Gouveia, Hueber, R., Melzener, L., Messmer, T., Papadopoulos, J., Pimenta, J., Raina, D., Lieke Schaeken, Shirley, S., Bouchet, B.P. and Flack, J.E. (2023). Annual Review of Animal Biosciences, 12(1). https://doi.org/10.1146/annurev-animal-021022-055132.
How biofabrication can accelerate cultured meat’s path to market
Heine, S., Ahlfeld, T., Albrecht, F.B. et al. (2024) Nat Rev Mater 9, 83–85. https://doi.org/10.1038/s41578-024-00650-9